Chapter 9 Population Distribution and Abundance
Standing on a headland in central California
over looking the Pacific Ocean, a small group of students spots a group
of gray whales, Eschrichtilus robustus, rising to the surface and spouting water as they
swim northward (fig.9.la). The whales are rounding the point of land
their way to feeding grounds off the coasts of
the grove of pine trees on the headland where
the students stand gazing at the whales is winter home to another long
distance traveler:monarch butterflies,Danaus plexippus(fig.9.b).The lazy flying of the bright orange and black monarch
butterflies gives no hint of their capacity to migrate.Some of the butterflies flew to the grove of pines the
previous autumn from as far away as the Rocky Mountains of southern
Canada.As the students watch the whales.the male monarch butterflies pursue and mate with the
female monarch butterflies.After mating.The males
die, while the females begin a migration that leads inland and north.The females stop to lay eggs on any milkweeds they encounter
along the way and eventually die; however their off spring continue
the migration.the monarch caterpillars grow quickly on their
diet of milkweed and then transform to pupae contained within cocoons.The monarch butterflies that emerge from the cocoons
mate and,like the previous generation,fly northward and inland.By moving
farther north and inland each generation, some of the monarch butterflies
eventually reach the Rocky Mountains of southern Canada.far from
where their ancestors fluttered around the group of students on the
pine-covered coastal headland.

Figure 9.1 (a)During
their annual migration,the entire population of gray
whales migrates from subtropical waters
Then as the autumn days grow shorter.the monarch butterflies begin their long flight back
to the coastal grove of pines.This autumn generation.which numbers in the millions.Flies southwest to their wintering grounds on the coast
of central and southern California.Some of them
might fly over
Gray whales and monarch butterflies, as diffluent
as they may appear, lead parallel lives.The Monterey
pines,Pinus radiata, covering
the headland where the monarch butterflies overwinter
and by which the gray whales pass twice each year are quite different.The Monterey pine population does not migrate each generation
and has a highly restricted distribution.The
current natural range of the Monterey pine is 1imited to a few sites
on the coast of central and northern California and to two islands 0ff
the coast of western Mexico.These scattered populations are the remnants
of a large continuous population that extended for over
With these three examples.we begin to consider the ecology of populations.Ecologists usually define a population as a group of individuals of a single species inhabiting
a specific area.A population of plants or animals might occupy
a mountaintop.a river basin,a coastal
marsh,or an island.All areas
defined by natural boundaries.Just as often, the populations studied by
biologists occupy artificially defined areas such as a particular country,
country, or national park.The areas inhabited by populations range in
size from the few cubic centimeters occupied by the bacteria in a rotting
apple to the millions of square kilometers occupied by a population
of migratory whales.A population studied by ecologists may consist
of a highly localized group of individuals representing fraction of
the total population of a species.or it may consist of all the individuals of
a species across its entire range.
Ecologists study populations for many reasons.
Population studies hold the key to saving endangered species, controlling
pest populations, and managing fish and game populations. They also
offer clues to understanding and controlling disease epidemics. Finally,
the greatest environmental challenge to biological diversity and the
integrity of the entire biosphere is at its heart a population problem—the
growth of the human population.
All populations share several characteristics.
The first is its distribution. The distribution of a population includes
the size, shape, and location of the area it occupies. A population
also has a characteristic pattern of spacing of the individuals within
it. It is also characterized by the number of individuals within it
and their density, which
is the number of individuals per unit area. Additional characteristics
of populations—their age distributions, birth and death rates, immigration
and emigration rates, and rates of growth—are the subject of chapters
10 and 11. In chapter 9 we focus on two population characteristics:
distribution and abundance.
CONCEPTS
l
The physical environment limits
the geographic distribution of species.
l
On small scales, individuals
within populations are distributed in patterns that may be random, regular,
or clumped; on larger scales, individuals within a population are clumped.
l
Population density declines
with increasing organism size.
l
Rarity is influenced by geographic
range, habitat tolerance, and population size; rare species are vulnerable
to extinction.
CASE HISTORIES: distribution
limits
The physical environment limits
the geographic distribution of species.
A major theme
in chapters 4, 5, and 6 is that individual organisms have evolved physiological,
anatomical, and behavioral characteristics that compensate for environmental
variation. Organisms compensate for temporal and spatial variation in
the environment by regulating body temperature and water content and
by foraging in a way that maintains energy intake at relatively high
levels. However, there are limits on how much organisms can compensate
for environmental variation.
While there are few environments on earth
without life, no single species can tolerate the full range of earth's
environments. For each species some environments are too warm, too cold,
too saline, or unsuitable in other ways. As we saw in chapter 6, organisms
take in energy at a limited rate. It appears that at some point, the
metabolic costs of compensating for environmental variation may take
up too much of an organism's energy budget. Partly because of these
energy constraints, the physical environment places limits on the distributions
of populations. Let's now turn to some actual species and explore the
factors that limit their distributions.
Kangaroo Distributions and Climate
The Macropodidea includes the kangaroos and wallabies, which are
some of the best known of the Australian animals. However, this group
of large-footed mammals includes many less familiar species, including
rat kangaroos and tree kangaroos. While some species of macropods
can be found in nearly every part of
G. Caughley and
his colleagues (1987) found a close relationship between climate and
the distributions of the three largest kangaroos in

FIGURE 9.2 Climate and the distributions
of three kangaroo species (data from Caughley
et al. 1987).
The distributions of these three large kangaroo
species cover most of
Regardless of how the influences of climate
are played out, the relationship between climate and the distributions
of species can be stable over long periods of time. The distributions
of the eastern grey, western grey, and red
kangaroos have been stable for at least a century. In the next example,
we discuss a species of beetle that appears to have maintained a stable
association with climate for 10,000 to 100,00
years.
A Tiger Beetle of Cold Climates
Tiger beetles
have entered our discussions several times. In chapter 4, we saw how
one species regulates body temperatures on hot black beaches in
The tiger beetle Cicindela
longilabris lives at higher latitudes and
higher elevations than just about any other species of tiger beetle
in
the maritime provinces of eastern
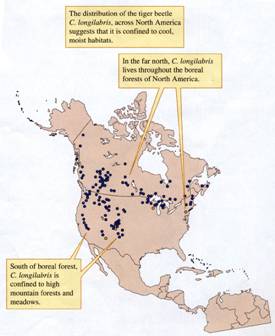
FIGURE
(data from Schultz Quinlan, and Hadley 1992).
Ecologists suggest that during the last glacial
period C. longilabris lived far south of its
present range limits. Then with climatic warming and the retreat of
the glaciers, the tiger beetles followed their preferred climate northward
and up in elevation into the mountains of western
Intrigued by the distribution and history
of C. longilabris, Thomas Schultz, Michael
Quinlan, and Neil Hadley (1992) set out to study the environmental physiology
of widely separated populations of the species. Populations separated
for many thousands of years may have been exposed to significantly different
environmental regimes. If so, natural selection could have produced
significant physiological differences among populations. The researchers
compared the
physiological characteristics of beetles from populations
of C. longilabris from
Schultz and his colleagues found that the
metabolic rates of C. longilabris are higher
and its preferred temperatures lower than those of most other tiger
beetle species that have been studied. These differences support the
hypothesis that C. longilabris is adapted to the cool climates of boreal and
montane forests. In addition, the researchers found that none
of their measurements differed significantly among populations of C.
longilabris. Figure 9.4 illustrates the remarkable similarity
in preferred body temperature shown by foraging C. longilabris from populations separated by as much as
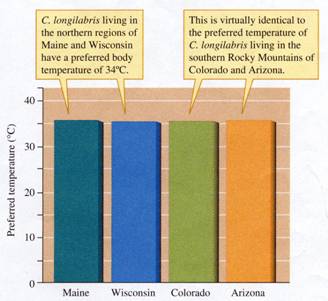
FIGURE 9.4 Uniform temperature
preference across an extensive geographic range (data from Schuttz, Quinlan, and Hadley 1992).
Now, let's consider how the physical environment
may limit the distribution of plants. Our example is drawn from the
arid and semiarid regions of the American Southwest.
Distributions of Plants along a Moisture-Temperature
Gradient
In chapter
4, we discussed the influence of pubescence on leaf temperature in plants
of the genus Encelia. Variation in leaf pubescence
among Encelia species appears to correspond directly to the distributions
of these species along a moisture-temperature gradient from the

FIGURE
9.5 The distributions of four Encelia
species in southwestern
These geographic limits to these species'
distributions correspond to variations in temperature and precipitation.
The coastal environments where E. californica
lives are all relatively cool. However, average annual precipitation
differs a great deal across the distribution of this species. Annual
precipitation ranges from about
Variation in leaf pubescence does not correspond
entirely to the macroclimates inhabited by Encelia
species, The leaves of E. frutescens
are nearly as free of pubescence as the coastal species E. californica.
However, E. frutescens grows side by side
with E. farinosa in some of the hottest deserts in the world. Because
they are sparsely pubescent, the leaves of E. frutescens
absorb a great deal more radiant energy than the leaves of E. farinosa (fig. 9.6). Under similar conditions, however, leaf
temperatures of the two species nearly identical. How does E. frutescens avoid overheating? The leaves do not overheat because
they transpire at a high rate and are evaporatively
cooled as a consequence.

FIGURE 9.6 Light absorption by leaves of Encelia frutescens and E. farinosa (data
from Ehleringer and Clark 1988).
Evaporative cooling solves one ecological
puzzle appears to create another. Remember that these two shrubs live:
in some of the hottest and driest deserts in the world. Where does E.
frutescens get enough water to evaporatively
cool its Leaves? Though the distributions of E. frutescens
and E. farinosa overlap a great deal on a
geographic scale, these two species occupy distinctive microenvironments.
As shown in figure 9.7, E. farinosa grows
mainly on upland slopes, while E. frutescens
is largely confined to ephemeral stream channels, or desert washes.
Along washes, runoff combined with deep soils increases the availability
of soil moisture. This example reminds us of a principle that we first
considered in chapter 4: organisms living in the same macroclimate may,
because of slight differences in local distribution, experience substantially
different microclimates. This is certainly true of the two barnacle
species we consider in the following example.

FIGURE 9.7 Temperature regulation and
distributions of Encelia farinosa
and E. frutescens across microenvironments.
Distributions of Barnacles along an Intertidal Exposure Gradient
The marine
intertidal zone presents a steep gradient of physical conditions
from the shore seaward. As we saw in chapter 3, the organisms high in
the intertidal zone are exposed by virtually
every tide while the organisms that live at lower levels in the intertidal
zone are exposed by the lowest tides only. Exposure to air differs at
different levels within the intertidal zone.
Organisms that live in the intertidal zone
have evolved different degrees of resistance to drying, a major factor
contributing to zonation among intertidal organisms
(see fig. 3.17).
Barnacles, one of the most common intertidal organisms, show distinctive patterns of zonation within the intertidal zone.
For example, Joseph Connell (1961) described how along the coast of

FIGURE 9.8 Distributions of two barnacle
species within the intertidal tone (data from
Connell 1961).

FIGURE 9.9 Barnacle mortality in the upper intertidal
zone (data from ConneU 1961).
Vulnerability to dessication,
however, does not completely explain the pattern of intertidal
zonation shown by Balanus
and Chthamalus. What excludes Chthamalus
from the lower intertidal zone? Though
the larvae of this barnacle settle in the lower intertidal
zone, the adults rarely survive there. Connell explored this
question by transplanting adult Chthamalus
to the lower intertidal zone and found that transplanted adults survive
in the lower intertidal zone very well. If
the physical environment does not exclude Chthamalus
from the lower intertidal zone, what does?
It turns out that this species is excluded from the lower intertidal
zone by competitive interactions with Balanus.
We discuss the mechanisms by which this competitive exclusion is accomplished
in chapter 13, which covers interspecific
competition.
These barnacles remind us that the environment
consists of more than just physical and chemical factors. An organism's
environment also includes biological factors, in many situations,
biological factors may be as important or even more important than physical
factors in determining the distribution and abundance of species. Often
the influences of biological factors remain hidden, however, because
of the difficulty of demonstrating them. In ecology, we must usually
probe deeper to see beyond outward appearances, as Connell did when
he transplanted Chthamalus from the upper to the lower intertidal
zone. The influence of biological factors, such as competition, predation,
and disease, on the distribution and abundance of organisms is a theme
that enters our discussions frequently in the remainder of this book,
especially in chapters13.14, and15.
CASE HISTORIES:distribution
patterns
On small scales, individuals within populations are distributed
in patterns that may be random, regular, or clumped; on larger scales,
individuals within a population are clumped.
We have just
considered how the environment limits the distributions of species.
When you map the distribution of a species such as the red kangaroo
in
Ecologists refer frequently to large-scale
and small-scale phenomena. What is "large" or "small"
depends on the size of organism or other ecological phenomenon under
study. For this discussion, small
scale refers to distances of no more than a few hundred meters,
over which there is little environmental change significant to the organism
under study. Large scale refers to areas over which
there is substantial environmental change. In this sense, large scale
may refer to patterns over an entire continent or patterns along a mountain
slope, where environmental gradients are steep. Let's begin our discussion
with patterns of distribution observed at small scales.
Distributions of Individuals on Small Scales
Three basic
patterns of distribution are observed on small scales: random, regular,
or clumped. A random distribution is one in which individuals
within a population have an equal chance of living anywhere within an
area. A regular distribution
is one in which individuals are uniformly spaced. In a clumped distribution, individuals have a much higher probability of
being found in some areas than in others (fig. 9.10).

FIGURE 9.10 Random, regular, and clumped
distributions.
These three basic patterns of distribution
are produced by the kinds of interactions that take place between individuals
within a population, by the structure of the physical environment, or
by a combination of interaction and environment each other, repel each
other, or ignore each other. Mutual attraction creates clumped, or aggregated,
patterns of distribution. Regular patterns of distribution are produced
when individuals avoid each other or claim exclusive use of a patch
of landscape. Neutral responses contribute to random distributions.
The patterns created by social interactions
may be reinforced or damped by the structure of the environment. An
environment with patchy distributions of nutrients, nesting site, water,
and so forth fosters clumped distribution patterns. An environment with
a fairly uniform distribution of resources and frequent, random patterns
of disturbance (or mixing) tends to reinforce random or regular distributions.
Let's now consider factors that influence the distributions of some
species in nature.
Distributions of Tropical Bee Colonies
Stephen Hubbell
and Leslie Johnson (1977) recorded a dramatic example of how social
interactions can produce and enforce regular spacing in a population.
They studied competition and nest spacing in populations of stingless
bees in the family Trigonidae. The bees they
studied live in tropical dry forest in
Stingless bees are abundant in tropical and subtropical
environments, where they gather nectar and pollen from a wide variety
of flowers. They generally nest in trees and live in colonies made up
of hundreds to thousands of workers, Hubbell and Johnson observed that
some species of stingless bees are highly
aggressive to other members of their species from other colonies, while
others are not. Aggressive species usually forage in groups and feed
mainly on flowers that occur in high-density clumps. The nonaggressive
species feed singly or in small groups and on more widely distributed
flowers.
Hubbell and Johnson studied several species
of stingless bees to determine whether there
is a relationship between aggressiveness and patterns of colony distribution.
They predicted that the colonies of aggressive species would show regular
distributions while those of nonaggressive
species would show random or clumped distributions, They concentrated
their studies on a
Though Hubbell and Johnson were interested
in how bee behavior might affect colony distributions, they recognized
that the availability of potential nest sites for colonies could also
affect distributions. So, in one of the first steps in their study,
they mapped the distributions of trees suitable for nesting. They found
that potential nest trees were distributed randomly through the study
area and that the number of potential nest sites was much greater than
the number of bee colonies. What did these measurements tell the researchers?
They indicated that the number of colonies in the study area was not
limited by availability of suitable trees and that clumped and regular
distribution of colonies would not be due to an underlying clumped or
regular distribution of potential nest sites,
Hubbell and Johnson were able to map the nests
of five of the nine species of stingless bees
accurately, The nests of four of these species were distributed
regularly. As they had predicted, all four species with regular nest
distributions were highly aggressive to bees from other colonies of
their own species, The fifth species, Trigona
dorsalis, was not aggressive and its nests were randomly distributed
over the study area. Figure 9.11 contrasts the random distribution of
T. dorsalis with the regular distribution
of one of the aggressive species, T. fulviventris.
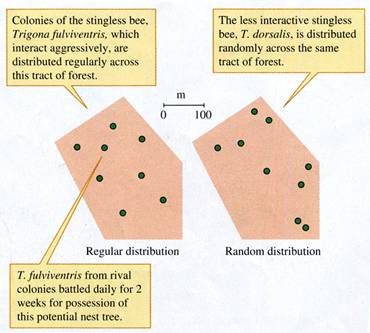
FIGURE 9.11 Regular and random distributions
of stingless bee colonies in the tropical
dry forest (data from Hubbell and Johnson 1977).
The researchers also studied the process by
which the aggressive species establish new colonies. In the process,
they made observations that provide insights into the mechanisms that
establish and maintain the regular nest distributions of species such
as T. fulviventris. This species and the other aggressive species
apparently mark prospective nest sites with a pheromone. Pheromones are chemical substances secreted by some animals for communication
with other members of their species, The pheromone secreted by these
stingless bees attracts and aggregates members
of their colony to the prospective nest site; however, it also attracts
workers from other nests.
If workers from two different colonies arrive
at the prospective nest, they may fight for possession. Fights may be
escalated into protracted battles. Hubbell and Johnson observed battles
over a nest tree that lasted for 2 weeks. Each dawn, 15 to 30 workers
from two rival colonies arrived at the contested nest site. The workers
from the two rival colonies faced off in two swarms and displayed and
fought with each other. In the displays, pairs of bees faced each other,
slowly flew vertically to a height of about
Distributions of Desert Shrubs
Half a century
ago desert ecologists suggested that desert shrubs tend to be regularly
spaced due to competition between the shrubs. You can see the patterns
that inspired these early ecologists by traveling across the seemingly
endless expanses of the Mojave Desert in western

FIGURE 9.12 Are local populations of the
creosote bush. Larrea tridentata,
distributed reguarly?
Quantitative sampling and statistical analysis
of the distributions of creosote bushes and other desert shrubs led
to a controversy that took the better part of two decades to settle.
In short, when different teams of researchers quantified the distributions
of desert shrubs, some found the regular distributions reported by earlier
ecologists. Others found random or clumped distributions. Still others
reported all three types of distributions.
Though we are generally accustomed to having one answer
to our questions, the answers to ecological questions are
often more complex. Research by Donald Phillips and James MacMahon
(1981) showed that the distribution of creosote bushes changes as they
grow. They mapped and analyzed the distributions of creosote bushes
and several other shrubs at nine sites in the Sonoran
and
The results of this study indicate that the
distribution of desert shrubs changes from clumped to random to regular
distribution patterns as they grow. The young shrubs tend to be clumped
for three reasons: because seeds germinate at a limited number of "safe
sites," because seeds are not dispersed far from the parent plant,
or because asexually produced offspring are necessarily close to the
parent plant. Phillips and MacMahon proposed
that as the plants grow, some individuals in the clumps die, which reduces the
degree of clumping. Gradually, the distribution of shrubs becomes more
and more random. However, competition among the remaining plants produces
higher mortality among plants with nearby neighbors, which thins the
stand of shrubs still further and eventually creates a regular distribution
of shrubs. This hypothetical process is summarized in figure 9.13.
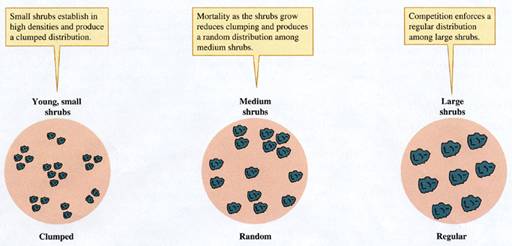
FIGUIRE 9.13 Hypothetical change in shrub
distributions with increasing shrub size.
Phillips and MacMahon
and other ecologists proposed that desert shrubs compete for water and
nutrients, a competition that takes place belowground. How can we study
these belowground interactions? Work by Jacques Brisson
and James Reynolds (1994) provides a quantitative picture of the belowground
side of creosote bush distributions. These researchers carefully excavated
and mapped the distributions of 32 creosote bushes in the
The 32 excavated creosote bushes occupied
a 4 by
The complex pattern of root distributions
uncovered confirmed the researchers proposal:
Creosote bush roots grow in a pattern that reduces overlap between the
roots of adjacent plants (fig.
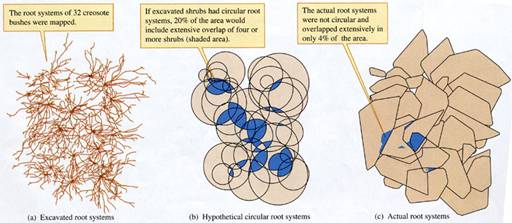
FIGURE 9.14 Creosote bush root distributions:
hypothetical versus actual root overlap (data from Brisson and Reynolds 1994).
After more than two decades of work on this
single species of plant, desert ecologists have a much clearer understanding
of the factors that influence the distribution of individuals on a small
scale. On small scales, the creosote bush may have clumped, random,
or regular distributions. Hubbell and Johnson (1977) showed that stingless
bee colonies may also show different patterns of distribution, depending
on the level of aggression between colonies. As we shall see in the
following section, however, on larger scales, individuals have clumped
distributions.
Distributions of Individuals on Large Scale
We have considered
how individuals within a population are distributed on a small scale:
how bee colonies are distributed within a few acres of forest and how
shrubs are distributed within a small stand. Now let's step hack and
ask how individuals within a population are distributed on a larger
scale over which there is significant environmental variation. For instance,
how does the density of individuals vary across the entire range of
a species? Is population density fairly regularly distributed across
the entire area occupied by a species, or are there a few centers of
high density surrounded by areas in which the species is present but
only in low densities?
Bird Populations Across
Terry Root
(1988) mapped patterns of bird abundance across
Root's analysis centers around
a series of maps that show patterns of distribution and population density
for 346 species of birds that winter in the
The fish crow population, though much more
restricted than that of the American crow, is also concentrated in a
few areas (fig. 9.15b). Fish crows are restricted to areas of open water
near the coast of the Gulf of Mexico and along the southern half of
the Atlantic coast of the

FIGURE 9.15
(a) Winter distribution of the American crow, Corvus brachyrynchos (b) winter
distribution of the fish crow. C. ossifragus
(data from Root 1988).
Might bird populations have clumped distributions
only on the wintering grounds? James H. Brown, David Mehlman, and George Stevens (1995) analyzed large-scale patterns
of abundance among birds across
Like Root, Brown and his colleagues found
that a relatively small proportion of study sites yielded most of the
records of each bird species. That is, most individuals were concentrated
in a fairly small number of hot spots. For instance, the densities of
red-eyed vireos are Iow in most places (fig. 9,16). Clumped
distributions were documented repeatedly. When the numbers of birds
across their ranges were totaled, generally about 25% of the locations
sampled supported over half of each population. By combining the results
of Root and Brown and his colleagues we can say confidently that at
larger scales, bird populations in

FIGURE 9.16 Red-eyed
vireos, Vireo olivaceus, counted along census
routes of the Breeding Bird Survey (data from Brown. Mehlman, and Stevens 1995)
Brown and his colleagues propose that these
distributions are clumped because the environment varies and individuals
aggregate in areas where the environment is favorable. What might be
the patterns of distribution for populations distributed along a known
environmental gradient? Studies of plant populations provide interesting
insights.
Plant Abundance Along Moisture
Gradients
Several decades
ago, Robert Whittaker gathered information on the distributions of woody
plants along moisture gradients in several mountain ranges across
Let's look at the distributions of some tree
species along moisture gradients in two of the mountain ranges studied
by Whittaker. Robert Whittaker and William Niering
(1965) studied the distribution of plants along moisture and elevation
gradients in the
There is a moisture gradient from the moist
canyon bottoms up the dry southwest-facing slopes. Whittaker and Niering found that along this gradient the Mexican pinyon pine, Pinus cembroides, is at its peak abundance on the uppermost and
driest part of the southwest-facing slope (fig. 9.17). Along the same
slope,

FIGURE. 9.17 Abundances of three tree species
on a moisture gradient in the
Whittaker (1956) recorded analogous tree distributions
along moisture gradients in the Great Smoky Mountains of eastern North
America, Again, the gradient was from a moist valley bottom to a drier
southwest-facing slope, Along this moisture gradient, hemlock, Tsnga canadensis, was concentrated
in the moist valley bottom and its density decreased rapidly upslope
(fig. 9.18). Meanwhile red maple, Acer rubrum.
grew at highest densities in the middle section
of the slope, while table mountain pine, Pinus
pungens, was concentrated on the driest upper sections. As
in the

FIGURE 9. 18 Abundance of three tree species
on a moisture gradient in the Great
The distribution of trees along moisture gradients
seems to resemble the clumped distributional patterns of birds across
the North American continent but on a smaller scale. All species of
trees discussed here showed a highly clumped distribution along moisture
gradients, and their densities decreased substantially toward the edges
of their distributions. In other words, like birds, tree populations
are concentrated in hot spots.
In this section, we have reviewed patterns
of distribution within populations. We have seen that those patterns
vary from one population to another and may depend upon the scale at
which ecologists make their observations. Now we turn from patterns
of spatial variation within populations to compare the average densities
of different populations. Is there any way to predict the average population
density of populations? While it is not possible to make precise predictions,
the following examples show that population densities are very much
influenced by organism size.
CASE HISTORIES: organism size and population density
Population density declines with increasing organism
size.
If you estimate
the densities of organisms in their natural environments, you will find
great ranges. While bacterial populations in soils or water can exceed
l09 per cubic centimeter and phytoplankton densities often
exceed 106 per cubic meter, populations of large mammals
and birds can average considerably less than one individual per square
kilometer. What factors produce this variation in population density?
The densities of a wide variety of organisms are highly correlated with
body size. In general, densities of animal and plant populations decrease
with increasing size.
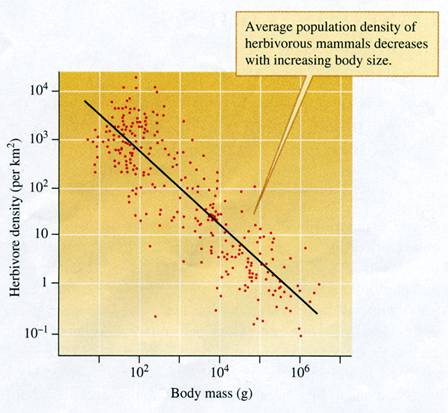
FIGURE 9.19 Body size and population density
of herbivorous mammals (data from Damuth 1981).
While it makes common sense that small animals
and plants generally live at higher population densities than larger
ones, quantifying the relationship between body size and population
density provides valuable information. First, quantification translates
a general qualitative notion into a more precise quantitative relationship.
For example, you might want to know how much population density declines
with increased body size. Second, measuring the relationship between
body size and population density for a wide variety of species reveals
different relationships for different groups of organisms. Differences
in the relationship between size and population density can be seen,
among major groups of animals.
Animal Size and Population Density
John Damuth (1981) produced one of the first clear demonstrations
of the relationship between body size and population density. He focused
his analysis on herbivorous mammals. The size of herbivorous mammals
included in the analysis ranged from small rodents, with a mass of about
Building on Damuth's
analysis, Robert Peters and Karen Wassenberg
(1983) explored the relationship between body size and average population
density for a wider variety of animals. Their analysis included terrestrial
invertebrates, aquatic invertebrates, mammals, birds, and poikilothermic
vertebrates. They included animals representing a great range in size
and population density. Animal mass ranged from 10-11 to
about
If you look closely at the data in figure
9.20, however, it is clear that there are differences among the animal
groups. First, aquatic invertebrates of a given body size tend to have
higher population densities, usually one or two orders of magnitude
higher, than terrestrial invertebrates of similar size. Second, mammals
tend to have higher population densities than birds of similar size.
Peters and Wassenberg suggest that it may
be appropriate to analyze aquatic invertebrates and birds separately
from the other groups of animals.

FIGURE 9.20 Animal size and population
density (data from Peters and Wassenberg 1983).
The general relationship between animal size
and population density has held up under careful scrutiny and reanalysis.
Plant ecologists have found a qualitatively similar relationship in
plant populations, as we see next. Plant Size and Population Density
James White (1985) pointed out that plant ecologists have been studying
the relationship between plant size and population density since early
in the twentieth century. He suggests that the relationship between
size and density is one of the most fundamental aspects of population
biology. White summarized the relationship between size and density
for a large number of plant species spanning a wide range of plant growth
forms (fig. 9.21).

FIGURE 9.21 Plant size and population density
(data from White 1985).
The pattern in figure 9.21 illustrates that
as in animals, plant population density decreases with increasing plant
size. However, the biological details underlying the size-density relationship
shown by plants are quite different from those underlying the size-density
patterns shown by animals. The different points in figures 9.19 and
9.20 represent different species of animals, A
single species of tree, however, can span a very large range of sizes
and densities during its life cycle. Even the largest trees, such as
the giant sequoia, Sequoia gigantea, start
life as small seedlings. These tiny seedlings can live at very high
densities. As the trees grow, density declines progressively until the
mature trees live at low densities. We discuss this process, which is
called self-thinning, in chapter 13. Thus, the size-density relationship
changes dynamically within plant populations and also differs significantly
between populations of plants that reach different sizes at maturity.
Despite differences in the underlying processes, the data summarized
in figure 9.21 indicate a predictable relationship between plant size
and population density.
The value of such an empirical relationship,
whether for plants or animals, is that it provides a standard against
which we can compare measured densities and gives an idea of expected
population densities in nature. For example, suppose you go out into
the field and measure the population density of some species of animal.
How would you know if the densities you encounter are unusually high,
low, or about average for an animal of the particular size and taxon?
Without an empirical relationship such as that shown in figures 9.20
and 9.21 or a list of species densities, it would be impossible to make
such an assessment. One question that we might attempt to answer with
a population study is whether a species is rare. As we shall see in
the next section, "Rarity and Extinction," rarity is a more
complex consideration than it might seem at face value.
CASE HISTORIES: rarity and extinction
Rarity is influenced by geographic range, habitat tolerance,
and population size; rare species are vulnerable to extinction.
Viewed on
a long-term, geological timescale, populations come and go and extinction
seems to be the inevitable punctuation mark at the end of a species'
history. However, some populations seem to be more vulnerable to extinction
than others. What makes some populations likely to disappear, while
others persist through geological ages? At the heart of the matter are
patterns of distribution and abundance. Species that are rare seem to
be more vulnerable to extinction. In order to understand and, perhaps,
prevent extinction, we need to understand the several forms of rarity.
Seven Forms Of Rarity and One
of Abundance.
Deborah Rabinowitz (1981) devised a classification of commonness and
rarity, based on combinations of three factors: (1) the geographic range
of a species (extensive versus restricted), (2) habitat tolerance (broad
versus narrow), and (3) local population size (large versus small).
Habitat tolerance is related to the range of conditions in which a species
can live. For instance, some plant species can tolerate a broad range
of soil texture, pH, and organic matter content, while other plant species
are confined to a single soil type. As we shall see, tigers have broad
habitat tolerance; however, within the tiger's historical range in Asia
lives the snow leopard, which is confined to a narrow range of conditions
in the high mountains of the Tibetan Plateau. Small geographic range,
narrow habitat tolerance, and low population density are attributes
of rarity.
As shown in figure 9.22, there are eight possible
combinations of these factors, seven of which include at least one attribute
of rarity. The most abundant species and those least threatened by extinction
have extensive geographic ranges, broad habitat tolerances, and large
local populations at least somewhere within their range. Some of these
species, such as starlings,

FIGURE 9.22 Commonness, rarity, and vulnerability
to extinction.
Let's look at species that represent the two
extremes of Rabinowitz's seven forms of rarity.
The first two discussions concern species that are rare according to
only one attribute. These are species that, before they become extinct,
may seem fairly secure. The final discussion concerns the very rarest
species, which show all three attributes of rarity. Though these rarest
species are the most vulnerable to extinction, rarity in any form appears
to increase vulnerability to extinction.
Rarity I: Extensive Range, Broad Habitat Tolerance, Small
Local Populations
It is easy
to understand how people were drawn to the original practice of falconry.
The sight and sound of a peregrine falcon, Falco
peregrinus, in full dive at over
DDT, which
produced thin eggshells and nesting failure, was enough to drive the
peregrine to the brink of extinction. Peregrine falcons were saved from
extinction by control of the use of DDT, strict regulation of the capture
of the birds, capfive breeding, and reintroduction of the birds to areas
where local populations had become extinct.

FIGURE 9.23 The peregrine falcon, Falco peregrinus, is found throughout
the Northern Hemisphere but lives at low population densities throughout
its range.
The range of the tiger, Panthera
tigris, once extended
from
Rarity II: Extensive Range, Large Populations, Narrow
Habitat Tolerance
When Europeans
arrived in
diminished and market hunters easily located and exploited
its remaining nesting sites, finishing off the remainder of the population.
By 1914, when the last passenger pigeon died in captivity, one of the
formerly most numerous bird species on earth was extinct. Extensive
range and high population density alone do not guarantee immunity from
extinction.
The rivers in the same region inhabited by
the passenger pigeon harbored an abundant, widely distributed but narrowly
tolerant fish, the harelip sucker, Lagochila
lacera. This fish was found in streams across most of the
east-central
Extreme Rarity: Restricted Range, Narrow Habitat Tolerance,
and Small Populations
Species that
combine small geographic ranges with narrow habitat tolerances and low
population densities are the rarest of the rare. This group includes
species such as the mountain gorilla, the giant panda, and the California
condor. Species showing this extreme form of rarity are clearly the
most vulnerable to extinction. Many island species have these attributes,
so it is not surprising that island species are especially vulnerable.
Of the 171 bird species and subspecies known to have become extinct
since 1600, 155 species have been restricted to islands. Of the 70 species
and subspecies of birds known to have lived on the
Organisms on continents that are restricted
to small areas, have narrow habitat tolerance,
and small population size are also vulnerable to extinction. Examples
of populations in such circumstances are common. More than 20 species
of plants and animals are confined to about
Amazingly, there are species with ranges even
more restricted than those of Ash Meadows,
Examples such as these fill books listing
endangered species. In nearly all cases, the key to a species' survival
is increased distribution and abundance. One of the most fundamental
needs for managing species, endangered or not, is making accurate estimates of
population size. Some of the conceptual and practical issues that population
ecologists must consider when censusing a
population are the subject of the Applications and Tools section.
APPLICATIONS AND TOOLS: estimating abundance --- from
whales to sponges
The abundance
of organisms and how abundance changes in time and space are among the
most fundamental concerns of ecology. These factors are so basic that
some authors define ecology as the study of distribution and abundance
of organisms. Because abundance is so important, ecologists should understand
how to estimate it for a wide variety of organisms. Keep in mind, however,
that ecologists do not measure abundance as an end in itself but as
a tool to understand the ecology of populations. Knowing how abundant
an organism is can tell us whether its population is growing, declining,
or stable. As we saw in the previous section, population size is one
of the characteristics that helps ecologists
assess a species' vulnerability to extinction. However, to estimate
the abundance of species the ecologist must contend with a variety of
practical challenges and conceptual subtleties. Some of these are discussed
here..
Estimating Whale Population Size
In 1989,
the journal Oceanus published a table that listed the estimated sizes
of whale populations. The table included the following note: "All
estimates . . . are highly speculative." Why is it difficult to
provide firm estimates of whale population size.'? Briefly, whales live
at low population densities and may be distributed across vast expanses
of ocean. They also spend much 6me submerged and move around a great
deal. As large as they are, you cannot count all the whales in the ocean.
instead, marine ecologists rely on population
estimation. Each method of estimation has its own limitations and uncertainties.
One method used to estimate population sizes
of elusive animals involves marking or tagging some known number of
individuals in the population, releasing the marked individuals so they
will mix with the remainder of the population, and then sampling the
population at some later time. The ratio of marked to unmarked individuals
in the sample gives an estimate of population size. The simplest formula
expressing this relationship is the Lincoln-Peterson index:
M/N = m/n
where:
M = the number of individuals marked
and released
N = the actual size of the study
population
m = the number of marked individuals
in a sample of the population
n = the total number of individuals
in the sample
The major
assumption of the Lincoln-Peterson index is that the ratio of marked
to unmarked individuals in the population as a whole equals the ratio
of marked to unmarked individuals in a sample of the population, ff
this is approximately so, then the population size is estimated as:
N = Mn/m
However. on average, the
Lincoln-Peterson index overestimates population size. To reduce this
tendency to overestimate, N. Bailey (1951, 1952) proposed a corrected
formula:
N=M(n+ I)/m+ 1
Some of the assumptions of mark and recapture
studies are:
l
All individuals in the population
have an equal probability of being captured.
l
The population is not increased
by births or immigration between marking and recapture.
l
Marked and unmarked individuals
die and emigrate at the same rates.
l
No marks are lost.
Although
real populations rarely meet ail these assumptions, mark and recapture
estimates of population size are often the best estimates available.
Whale populations have been
studied using mark and recapture techniques for some time. In the early
days of whale population studies, population biologists marked whales
by shooting a numbered metal dart into their blubber with a modified
shotgun. The idea was that the dart would be recovered when marked whales
were caught and processed during whaling operations. However, the accuracy
of this method was limited by several factors. First, it is difficult
to mark a free-swimming whale from a moving boat on the open sea, and
so biologists never knew exactly how many whales shot at were actually
marked. Second, the recovery of darts during processing was poor. It
is easy to overlook a relatively small dart on a huge whale. Experiments
showed that Japanese whalers recovered 60% to 70% of marks from whales
known to be marked, while other whaling fleets recovered an even lower
proportion. Still, the greatest limitation of this early mark and recapture
technique is that it requires killing whales during the recapture phase,
which is unacceptable when studying protected or endangered species,
Refined mark and recapture methods do not
require artificially marking or capturing whales. In the "marking"
phase of newer procedures, a whale is photographed and its distinguishing
marks are identified. These photographs, along with information such
as where the photograph was taken and whether the whale was accompanied
by an offspring, are catalogued for future reference. In the "recapture"
phase the whale is photographed at a later date and identified from
previous photos. This method is called photoidentification.
For more than two decades, Steven Katona (1989) has used photoidentification
to study die humpback whales, Megaptera novaeangliae, of the

FIGURE

FIGURE 9.25 Unique
markings identify individual humpback whales. A humpback whale called
"Siphon." #700, photographed in
Katona points out that the unique markings on a humpback whale's
flukes result from a combination of genetics
and accidents. In humpback whales, fluke pigmentation ranges from completely
black to white. This variation in pigmentation probably reflects genetic
differences between individuals. Injuries often produce sears that superimpose
other marks on the basic pigmentation of the flukes. Injuries may be
the result of fighting between humpback whales, attacks by sharks, or
attachment by parasites. The sears produced when these injuries heal
create black marks on white flukes and white marks on black flukes.
Using photographs of these marks, Katona and
his colleagues have produced the North Atlantic Humpback Catalog, which
includes photographs of more than 4,000 individual whales. The photographs
included in the catalog, along with information on where each photograph
was taken, whether the whale was accompanied by an
offspring, and other available observations, are curated for future reference. This photographic record is
an invaluable source of information for determining the migration mutes,
feeding grounds, breeding grounds, and size of the

FIGURE 9.26 Photo identification and the
From 1979 to 1986, Scott Baker, Janice Straley, and Anjanette Perry (1992)
photographed and identified 257 humpback whales along the coast of southeastern
N=M(n+l)/m+l=72(78+l)/22+l=247
In other words, Baker and his colleagues estimated
that there were 247 humpback whales in
Population ecologists have now applied the
photoidentification techniques to several whale species including
gray, right, blue, fin, sei, and killer whales.
For instance, by 1986, scientists had photographed about 200 of the
300 to 400 North Atlantic right whales remaining in the
Though it may be more challenging physically,
the process of counting whales is much like counting many other kinds
of animals such as humans, lynxes, trout, or ladybird beetles. However,
ecologists must use different methods to estimate the abundance of organisms
that have a more variable growth form or differ greatly in size. As
we shall see in the next example, this is particularly true when the
relative abundances of very different organisms are compared.
The Relative Abundance of Corals, Algae, and Sponges
The reefs
along the north coast of
changes on the reef would require detailed population
studies.
Fortunately, Terence Hughes (1996) had started
long-term studies of coral populations near
Hughes estimated percent cover by corals,
algae, and sponges on his study reef nearly every year from 1977 to
1993. He made his estimates from photographs of 12 study plots I m
Hughes' 16-year study shows clear changes
in the abundances of corals and algae (fig. 9.27). During the study,
algal cover increased 10-fold from 7% to 76%. At the same time, coral
cover decreased from about 48% to 13%, while sponge populations remained
fairly constant. Verifying the status of populations such as these is
one of the most basic aspects of ecological research. Such measurements
are the first step toward identifying the factors that determine the
distribution and abundance of organisms. With his photographs Hughes
was able to show that this shift from a coral-dominated reef to one
dominated by algae was due to increased mortality of coral colonies
and reduced recruitment of new corals to the population. These are aspects
of population dynamics that we cover in chapter 10.

FIGURE 9.27 Estimating abundance as percent
cover: corals, sponges. and algae (data from
Hughes 1996).
SUMMARY CONCEPTS
Ecologists
define a population as a group of individuals of a single species inhabiting
an area delimited by natural or human-imposed boundaries. Population
studies hold the key to solving practical problems such as saving endangered
species, controlling pest populations, or managing fish and game populations.
All populations share a number of characteristics. Chapter 9 focused
on two population characteristics: distribution and abundance.
While there are few environments on earth
without life, no single species can tolerate the full range of earth's
environments. Because all species find some environments too warm, too
cold, too saline, and so forth, the
physical environment limits the geographic distribution of species.
For instance, there is a close relationship between climate and the
distributions of the three largest kangaroos in
On
small scales, individuals within populations are distributed in patterns
that may be random, regular, or clumped. Patterns of distribution can be produced by the social
interactions within populations, by the structure of the physical environment, or by a combination of the two. Social organisms tend to be clumped; territorial organisms
tend to be regularly spaced.
An environment in which resources are patchy also fosters clumped distributions.
Aggressive species of stingless bees live in regularly distributed colonies,
while the colonies of nonaggressive species are randomly distributed. The distribution
of creosote bushes changes as they grow. On larger scales, individuals within a population are dumped. In
Population
density declines with increasing organism size. In general, animal population density declines with increasing body size. This negative relationship
holds for animals as varied as terrestrial invertebrates, aquatic invertebrates,
birds, poikilothermic vertebrates, and herbivorous
mammals. Plant population density also decreases with
increasing plant size. However, the biological details underlying
the size--density relationship shown by plants are quite different from
those underlying the size--density patterns shown
by animals. A single species of tree can span a very large range of sizes and densities during its life
cycle. The largest trees start
life as small seedlings that can live at very high population densities.
As trees grow, their population density declines progressively until
the mature trees live at low densities.
Rarity is influenced by geographic range,
habitat tolerance, and population size. Rarity of species can be expressed
as a combination of extensive versus restricted geographic range, broad
versus narrow habitat tolerance, and large versus small population size.
The most abundant species and those least threatened by extinction combine
large geographic ranges, wide habitat tolerance, and high local population
density. All other combinations of geographic range, habitat tolerance,
and population size include one or more attributes of rarity. Rare species
are vulnerable to extinction. Populations that combine restricted geographic
range with narrow habitat tolerance and small population size are the
rarest of the rare and are usually the organisms most vulnerable to
extinction.
The abundance of organisms and how abundance
changes in time and space are among the most fundamental concerns of
ecology. To estimate the abundance of species the ecologist must contend
with a variety of practical challenges and conceptual subtleties. Mark
and recapture methods are useful in the study of populations of active,
elusive, or secretive animals. Mark and recapture techniques, which
use natural distinguishing marks, are making an important contribution
to the study of populations of endangered whales. Ecologists studying
organisms, such as corals, algae, and sponges or many types of terrestrial
plants, that differ a great deal in size and
form often estimate abundance as coverage, the area covered by a species.
Patterns of distribution and abundance are ultimately determined by
underlying population dynamics.
REVIEW QUESTIONS
1. What confines
Encelia farinosa to upland slopes
in the
2. Spruce
trees, members of the genus Picea. occur
throughout the boreal forest and on mountains farther south, For example,
spruce grow in the Rocky Mountains south from the heart of boreal forest
all the way to the deserts of the southern
3. What kinds
of interactions within an animal population lead to clumped distributions?
What kinds of interactions foster a regular distribution? What kinds
of interactions would you expect to find within an animal population
distributed in a random pattern?
4. How might
the structure of the environment, for example, the distributions of
different soil types and soil moisture, affect the patterns of distribution
in plant populations? How should interactions among plants affect their
distributions?
5. Suppose
one plant reproduces almost entirely from seeds, and that its seeds
are dispersed by wind. and a second plant reproduces
asexually, mainly by budding from runners. How should these two different
reproductive modes affect local patterns of distribution seen in populations
of the two species?
6. Suppose
that in the near future, the fish crow population in
7. Use the
empirical relationship between size and population density observed
in the studies by Damuth (1981) (see fig.
9.19) and Peters and Wassenberg (1983) (see
fig. 9.20) to answer the following: For a given body size, which generally
has the higher population density, birds or mammals? On
average. which lives at lower population densities, terrestrial or aquatic
invertebrates? Does an herbivorous mammal twice the size of another
have on average one-half the population density of the smaller species?
Less than half? More than half?
8. Outline
Rahinowitz's classification (1981) of rarity, which she based
on size of geographic range, breadth of habitat tolerance, and population
size. In her scheme, which combination of attributes makes a species
least vulnerable to extinction? Which combination makes a species the
most vulnerable?
9. Can the
analyses by Damuth (1981) and by Peters and
Wassenberg (1983) be combined with that of Rabinowitz (1981) to make predictions about the relationship
of animal size to its relative rarity? What two attributes of rarity,
as defined by Rabinowitz, are not included
in the analyses by Damuth and by Peters and
Wassenberg?
10. Suppose
you have photoidantified 30 humpback whales
around the
SUGGESTED
Caughley, G., J. Short, G. C. Gfigg,
and H. Nix.
1987. Kangaroos and climate: an analysis of distribution. Journal of
Animal Ecology 56:751-61.
Ehleringer, J. R. and C. Clark. 1988. Evolution and adaptation in Encelia
(Asteraceae). In L. D. Gottlieb
and S. K. Jain., eds. Plant Evolutionary Biology.
Bfissou, J. and J. F. Reynolds. 1994. The effects of neighbors on root distribution
in a creosote bush (Larrea tridentota) population. Ecology 75:1693-702.
The paper by Brisson and Reynolds demonstrates extended studies of creosote
bush distributions belowground.
Phillips, D.
L. and J. A. MacMahon. 1981. Competition and spacing patterns in desert shrubs.
Journal of Ecology 69:97-115.
The paper by Phillips and MacMahon gives an excellent summary of the history and apparent
resolution of a controversy surrounding distributions of creosote bush.
Brown, J. H., D. W. Mehlman, and G. C. Stevens. 1995. Spatial variation in abundance.
Ecology 76:2028-43.
Root, T. 1988. Atlas of Wintering North American Birds.
Damuth, J. 1981. Population density and body size
in mammals. Nature 290:699-700.
Peters, R. H.
and K. Wassenberg. 1983. The effect of body size on animal abundance. Oecologia 60:89-96.
These are benchmark
papers on the relationship between animal size and population density.
Rabinowitz, D.,
This paper provides
an introduction and application of the concept of rarity developed by
Deborah Rabinowitz.
Baker. C. S., J. M. Straley,
and A. Perry. 1992. Population characteristics of individually identified
humpback whales in southeastern
Katona, S.K. 1989. Getting to
know you. Oceanus 32:37-44.
These papers provide
detailed accounts of using photoidentification
to study humpback whale populations.
ON THE NET
Visit our website at http://www,
mhhe.com/ecology for links to the following topics:
Animal Population
Ecology
Population Density
of Animals
Biodiversity
Endangered Species
Legislation Regarding Endangered Species